Fanny’s Factcheck: What is uranium-235?
From the comic book: 'De Kiekeboes: Uranium-235'
Flanders' most popular comic family – De Kiekeboes – has explored the whole world: from exotic places to deep in the ocean, and even the planetoid Spih. But now, it's daughter Fanny's turn to take the reins. In the latest comic album, she dives into the nuclear sector. The title "Uranium-235" already gives you a hint, but what exactly does that mean?
© In collaboration with De Standaard Uitgeverij. All illustrations and storylines belong to them.

Uranium in a nutshell
If you take a look at the Mendeleev's periodic table, you'll find the element ‘uranium’ somewhere near the bottom. We represent this with the symbol ‘U’ and it has the atomic number 92. It belongs to the group of elements discovered relatively recently in history: those known as the actinides. Uranium is – just like other actinides – ‘radioactive’.
Natural element
Uranium is a heavy metal that occurs naturally on Earth. While it appears rare, it is actually one of the most commonly occurring elements in the Earth's Crust. It is present everywhere in small quantities – in water, the soil, food and even our bodies. We also encounter uranium in low concentrations in the ocean.
Did you know that uranium is 500 times more common on Earth than gold?

Uranium variants
An atom is made up of three 'building blocks': the proton, the neutron and the electron. The proton is relatively heavy and has a positive electrical charge. The neutron is also relatively heavy, but electrically neutral. The electron, on the other hand, is very light and has a negative electrical charge. The number of protons in the atomic nucleus determines the atomic number and thus its place in Mendeleev's periodic table. An atom is electrically neutral: this is why there are just as many electrons floating around an atomic nucleus as there are protons in the nucleus. The neutrons joining them in the atomic nucleus provide stability.
Chemical elements often come in different variants. These variants have the same number of protons and therefore share the same chemical properties, but the number of neutrons in their nucleus differs. This means they have a slightly different mass. In our technical jargon, we call these ‘isotopes’.
These isotopes may be natural, or may be man-made (e.g. in particle accelerators or nuclear reactors). Mother Nature, in any case, has made three isotopes of uranium for us:
-
Uranium-234
92 electrons
92 protons
142 neutrons -
Uranium-235
92 electrons
92 protons
143 neutrons -
Uranium-238
92 electrons
92 protons
146 neutrons

Nuclear energy
Nuclear power stations generate electricity by causing neutrons to collide with uranium atoms. The uranium nucleus catches that neutron, making the nucleus unstable and splitting it into two smaller atomic nuclei. In doing so, two to three extra neutrons are shot away with high energy. These may, in turn, hit other uranium atoms. The process thus repeats itself time after time, hence the term 'chain reaction'.
Nuclear fission releases energy. The energy released is transported away through the coolant in the reactor to generate steam. That steam drives a turbine, which in turn is connected to an alternator. It is the alternator that generates electricity.
Did you know that low-enriched uranium the size of a chicken egg weighs 1.1 kilogrammes? We can produce just as much electricity as 88 tons of coal with that. The energy production from that one 'uranium egg' is equal to 80,000 'coal eggs'.
Enriching uranium
Not every variant – ‘isotope’, remember? – of uranium is suited to fission. Uranium-235 is easy to fission if it comes into contact with a neutron. It prefers to catch slow neutrons, but in fact, any of neutron – slow or fast – will do. For uranium-238, the picture is different: it is only willing to 'do a dance' with fast neutrons.
Did you know that water slows downs neutrons in nuclear reactors?
That means our water-cooled reactors need uranium-235 to be able to maintain a chain reaction. Unfortunately, natural uranium consists of 99.3% uranium-238 and only 0.7% uranium-235.[1] This is too little to keep the majority of nuclear reactors running. Hence why we start artificially increasing the quantity of uranium-235. We call this process ‘enrichment’.
---------------------------------------
[1]And the third element, uranium-234? Its presence, at 0.0054%, is negligible.
In doing so, its presence can be increased to …
-

less than 19.75%
Low-enriched uranium
Uranium enriched to 19.75% – of known more informally as 20% – comes under the category of ‘low-enriched uranium’. Nuclear power stations like Doel and Tihange typically work with an enrichment level of 4.5%. For our kinds of reactors, this is an ideal concentration for maintaining a chain reaction.
-
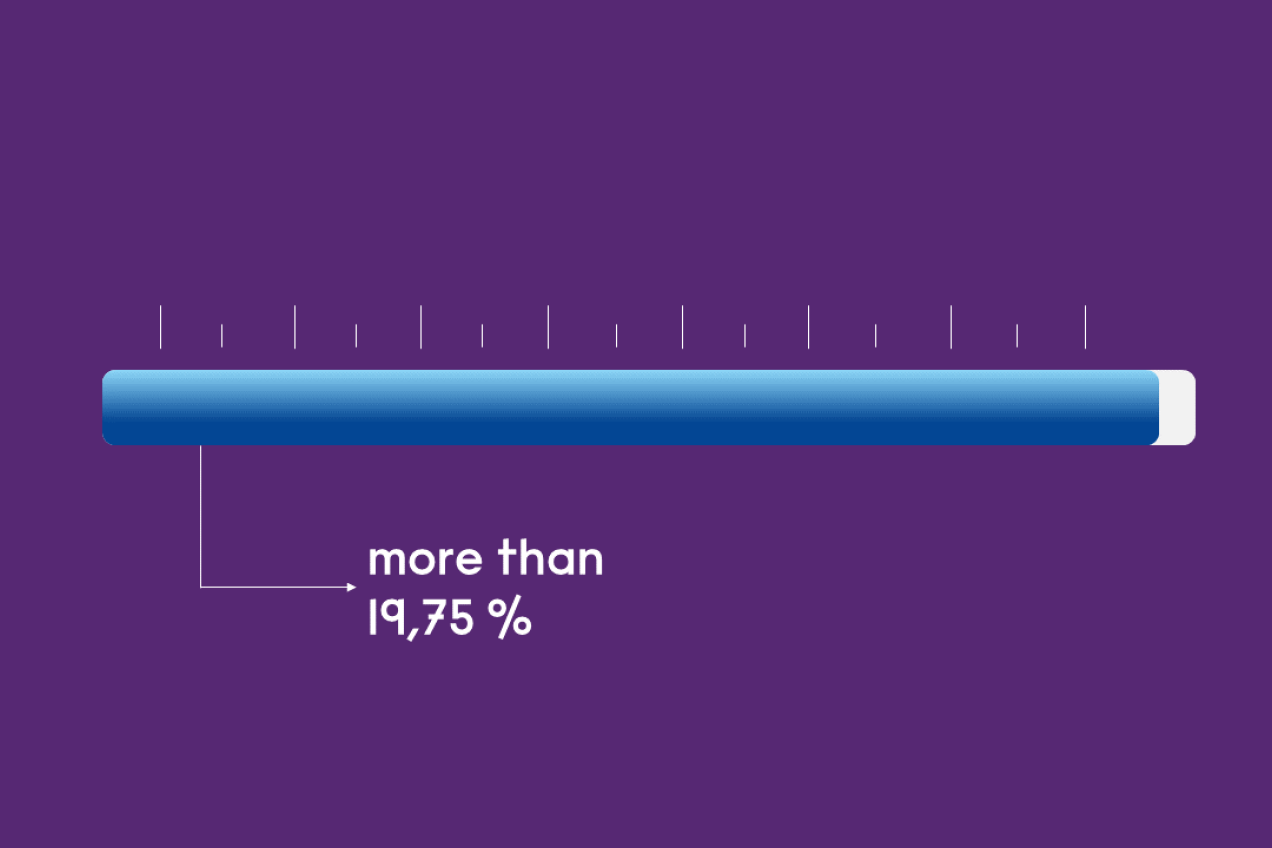
more than 20%
Highly enriched uranium
Uranium enriched by more than 19.75% is called highly enriched uranium. Globally, many efforts are being made to limit the use of this as far as possible, because above an enrichment level of 85%, it could be turned into an ingredient for nuclear weapons. At present, there are ten research reactors running on highly enriched uranium.

Safer world
Our BR2 research reactor has been working with highly enriched uranium since the 1960s too. This choice was made upon construction, such that the reactor would be able to deliver higher technical performance for material tests and production of medical radioisotopes. But this will not last much longer! We are planning to replace our fissile material with a low-enriched variant. This switchover is in our calendar for 2026. If everything goes to plan, we will then be making history once more. BR2 would then be the world's first research reactor with high-performance fissile material to make that step.
This switchover is a long process: years of development, extensive testing and a significant safety file will precede it. After all, we must prove to the Federal Agency for Nuclear Control (FANC) – the nuclear regulatory authority in Belgium – that the new fuel type is just as safe as the current one. Moreover, the research reactor must deliver the same technical performance using this new fuel type. And this performance is not to be sniffed at – BR2 is among the most powerful research reactors in the world. The medical and scientific sectors are counting on it. After all, it plays a vital global role in the production of medical radioisotopes and the testing of new materials and fuels for the nuclear industry.
In 2023, we ticked off a major milestone in this switchover process. For the first time, three low-enriched fuel test assemblies – just like their highly enriched brothers – demonstrated their capability and served as fissile material for the Belgian research reactor. The American ambassador, Michael M. Adler, praised us for this milestone [see video below].
Do you want to know how those testing fissile material elements passed the test?
Sustainable nuclear energy
Our current nuclear reactors work by splitting uranium-235, which is only a small part of the natural uranium cake. What if … we could also get energy from uranium-238? What if … in other words, we could also gain it from that 99.3% in natural uranium? Then, we would be able to get more energy from the same quantity of uranium and make more efficient use of our natural raw materials. We are handling this issue – as commissioned by the Belgian government – with research and development into our lead-cooled Small Modular Reactor (SMR).
Uranium-238 is only willing to split when it collides with fast neutrons. In order to keep neutrons fast, we need to have them collide with a coolant consisting of heavy atoms. The atoms in water are ‘too light’, so we chose lead for this reactor. The liquid metal allows for more efficient use of raw materials, but also has some other advantages. This type of reactor will contribute towards a safer and more sustainable form of nuclear energy.
Want to dive into those benefits?
Nuclear recycling
Our lead-cooled SMR will not only use raw materials more efficiently. Due to the fact that it operates with fast neutrons, the reactor will also have the potential to operate in a closed fissile material cycle and use MOX as a nuclear fuel. MOX stands for mixed oxide, which consists of an oxide of uranium and plutonium. It could be made from recycling spent fuel from our existing power plants on the one hand, or may in the future consist of recycling our own fissile material. We can recycle up to 96% of spent fuel again! This could heavily influence the way in which we handle spent fissile material from our current nuclear power stations today.
Do you want to know how to do that?